HS Dosing COSENTYX® (secukinumab)


FDA Approves Cosentyx for HS - Practical Dermatology
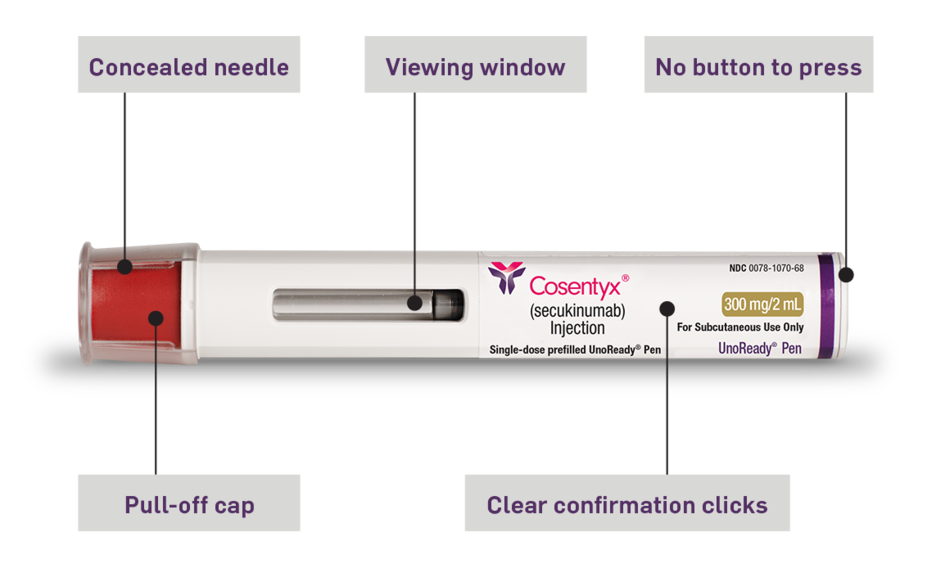
HS Dosing COSENTYX® (secukinumab)

Full article: Biologic Treatments of Psoriasis: An Update for the Clinician

FAQ, COSENTYX® (secukinumab)

Novartis's Cosentyx Received the US FDA's Approval for the Treatment of Hidradenitis Suppurativa

Cosentyx 150 mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC) - (emc)

These highlights do not include all the information needed to use COSENTYX safely and effectively. See full prescribing information for COSENTYX. COSENTYX® (secukinumab) injection, for subcutaneous or intravenous useInitial U.S. Approval: 2015

📢📢 BREAKING NEWS!!! 📰📰📰 FDA had approved ✅️ Secukinumab (Cosentyx

PDF) A Case of Moderate Hidradenitis Suppurativa and Psoriasis Treated with Secukinumab
Which treatment is safer for psoriasis between Humira (adalimumab) and Cosentyx (secukinumab) in the short and long term? - Quora
Cosentyx Dosage Guide

First IL-17 A Inhibitor for Hidradenitis Suppurativa Approved by FDA - LiVDerm

Rheumatology Dosing, COSENTYX® (secukinumab)

New indication for Cosentyx (secukinumab) biological: hidradenitis suppurativa